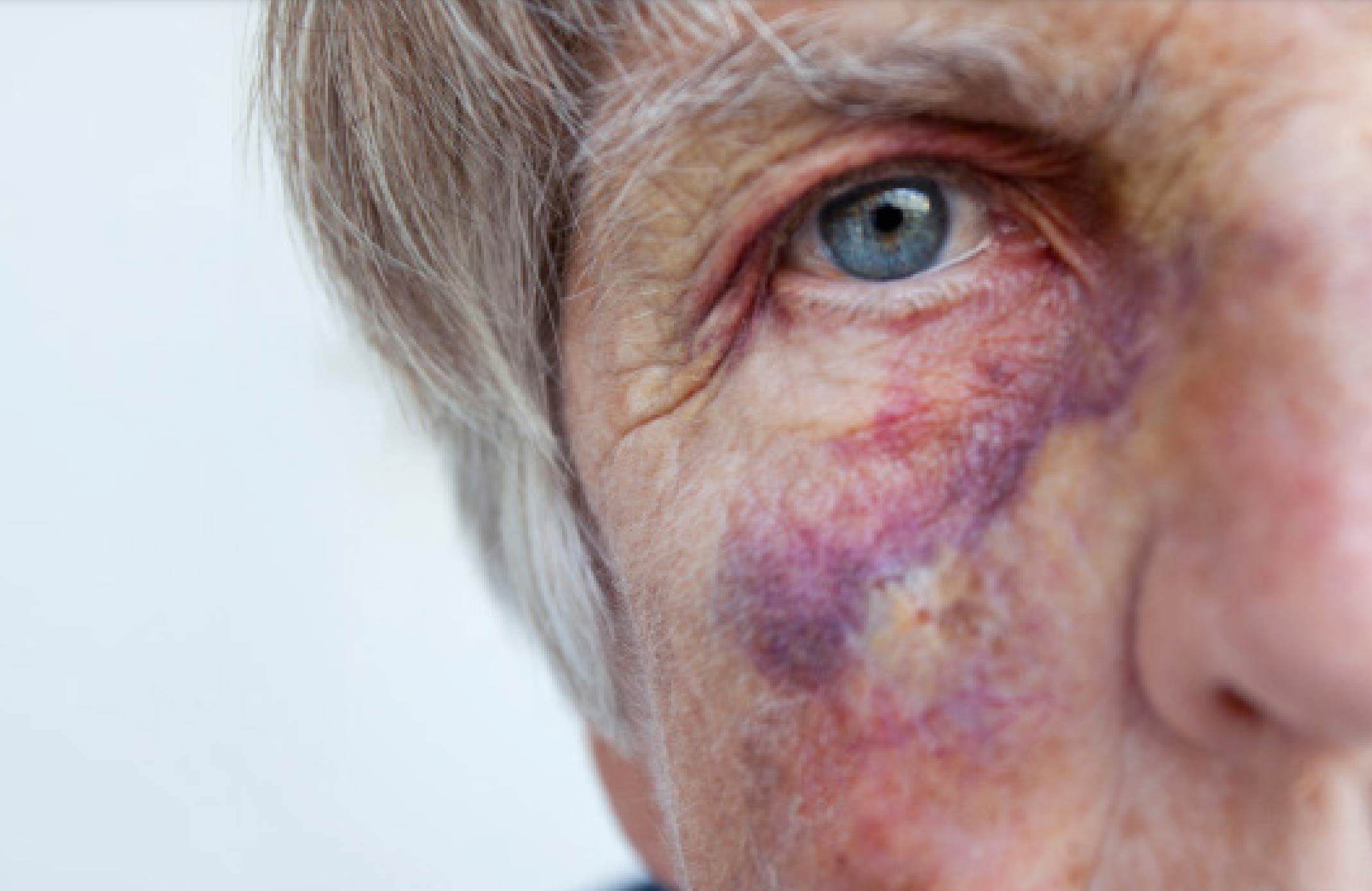
Healthcare & advertising
professionals united
against the influence of
marketing on medicine
Working together
to reduce medication costs,
improve outcomes & promote
safer prescribing


Meet RxBalance
Countering bias with evidence to change our prescribing culture